Page 73 - Impiantistica industriale
P. 73
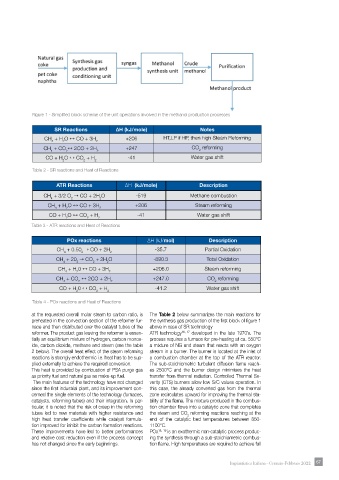
Figure 1 - Simplified block scheme of the unit operations involved in the methanol production processes
SR Reactions ΔH (kJ/mole) Notes
CH + H O ↔ CO + 3H +206 HT,LP if HP, then high Steam Reforming
4 2 2
CH + CO ↔ 2CO + 2H +247 CO reforming
4 2 2 2
CO + H O ↔ CO + H -41 Water gas shift
2 2 2
Table 2 - SR reactions and Heat of Reactions
ATR Reactions ΔH (kJ/mole) Description
CH + 3/2 O → CO + 2H O -519 Methane combustion
4 2 2
CH + H O ↔ CO + 3H +206 Steam reforming
4 2 2
CO + H O ↔ CO + H -41 Water gas shift
2 2 2
Table 3 - ATR reactions and Heat of Reactions
POx reactions ΔH (kJ/mol) Description
CH + 0.50 → CO + 2H -35.7 Partial Oxidation
4 2 2
CH + 20 → CO + 2H O -890.3 Total Oxidation
4 2 2 2
CH + H 0 ↔ CO + 3H +206.0 Steam reforming
4 2 2
CH + CO ↔ 2CO + 2H +247.0 CO reforming
4 2 2 2
CO + H 0 ↔ CO + H -41.2 Water gas shift
2 2 2
Table 4 - POx reactions and Heat of Reactions
at the requested overall molar steam to carbon ratio, is The Table 2 below summarizes the main reactions for
preheated in the convection section of the reformer fur- the synthesis gas production of the first block of figure 1
nace and then distributed over the catalyst tubes of the above in case of SR technology
reformer. The product gas leaving the reformer is essen- ATR technology 16, 17 developed in the late 1970’s. The
tially an equilibrium mixture of hydrogen, carbon monox- process requires a furnace for pre-heating at ca. 550°C
ide, carbon dioxide, methane and steam (see the table a mixture of NG and steam that reacts with an oxygen
2 below). The overall heat effect of the steam reforming stream in a burner. The burner is located at the inlet of
reactions is strongly endothermic i.e. heat has to be sup- a combustion chamber at the top of the ATR reactor.
plied externally to achieve the required conversion. The sub-stoichiometric turbulent diffusion flame reach-
This heat is provided by combustion of PSA purge gas es 2500°C and the burner design minimises the heat
as priority fuel and natural gas as make-up fuel. transfer from thermal radiation. Controlled Thermal Se-
The main features of the technology have not changed verity (CTS) burners allow low S/C values operation. In
since the first industrial plant, and its improvement con- this case, the already converted gas from the thermal
cerned the single elements of the technology (furnaces, zone recirculates upward for improving the thermal sta-
catalysts, reforming tubes) and their integration. In par- bility of the flame. The mixture produced in the combus-
ticular, it is noted that the risk of creep in the reforming tion chamber flows into a catalytic zone that completes
tubes led to new materials with higher resistance and the steam and CO reforming reactions reaching at the
2
high heat transfer coefficients while catalyst formula- end of the catalytic bed temperatures between 850-
tion improved for inhibit the carbon formation reactions. 1100°C.
These improvements have led to better performances POx is an exothermic non-catalytic process produc-
18, 19
and relative cost reduction even if the process concept ing the synthesis through a sub-stoichiometric combus-
has not changed since the early beginnings. tion flame. High temperatures are required to achieve full
Impiantistica Italiana - Gennaio-Febbraio 2022 67 67