Page 86 - Impiantistica industriale
P. 86
SUSTAINABLE SOLUTIONS
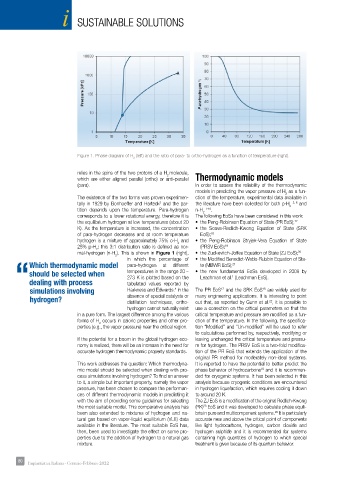
Figure 1. Phase diagram of H (left) and the ratio of para- to ortho-hydrogen as a function of temperature (right).
2
relies in the spins of the two protons of a H molecule,
which are either aligned parallel (ortho) or anti-parallel Thermodynamic models
2
(para). In order to assess the reliability of the thermodynamic
models in predicting the vapor pressure of H as a fun-
2
The existence of the two forms was proven experimen- ction of the temperature, experimental data available in
tally in 1929 by Bonhoeffer and Harteck and the par- the literature have been collected for both p-H 2 5, 6 and
3
tition depends upon the temperature. Para-hydrogen n-H 7-16 .
2
corresponds to a lower rotational energy, therefore it is The following EoSs have been considered in this work:
the equilibrium hydrogen at low temperatures (about 20 • the Peng-Robinson Equation of State (PR EoS); 17
K). As the temperature is increased, the concentration • the Soave-Redlich-Kwong Equation of State (SRK
of para-hydrogen decreases and at room temperature EoS); 18
hydrogen is a mixture of approximately 75% o-H and • the Peng-Robinson Stryjek-Vera Equation of State
2
25% p-H : this 3:1 distribution ratio is defined as nor- (PRSV EoS); 19
2
mal-hydrogen (n-H ). This is shown in Figure 1 (right), • the Zudkevitch-Joffee Equation of State (ZJ EoS); 20
2
in which the percentage of • the Modified Benedict-Webb-Rubbin Equation of Sta-
Which thermodynamic model para-hydrogen at different te (MBWR EoS);
21
should be selected when temperatures in the range 20 – • the new fundamental EoSs developed in 2009 by
273 K is plotted based on the
Leachman et al. (Leachman EoS).
1
“dealing with process tabulated values reported by
simulations involving Harkness and Edwards. In the The PR EoS and the SRK EoS are widely used for
4
17
18
hydrogen? absence of special catalysts or many engineering applications. It is interesting to point
out that, as reported by Gunn et al. , it is possible to
distillation techniques, ortho-
22
hydrogen cannot naturally exist use a correction on the critical parameters so that the
in a pure form. The largest difference among the various critical temperature and pressure are modified as a fun-
forms of H occurs in caloric properties and other pro- ction of the temperature. In the following, the specifica-
2
perties (e.g., the vapor pressure) near the critical region. tion “Modified” and “Un-modified” will be used to refer
to calculations performed by, respectively, modifying or
If the potential for a boom in the global hydrogen eco- leaving unchanged the critical temperature and pressu-
nomy is realized, there will be an increase in the need for re for hydrogen. The PRSV EoS is a two-fold modifica-
accurate hydrogen thermodynamic property standards. tion of the PR EoS that extends the application of the
original PR method for moderately non-ideal systems.
This work addresses the question: Which thermodyna- It is reported to have the potential to better predict the
mic model should be selected when dealing with pro- phase behavior of hydrocarbons and it is recommen-
19
cess simulations involving hydrogen? To find an answer ded for cryogenic systems. It has been selected in this
to it, a simple but important property, namely the vapor analysis because cryogenic conditions are encountered
pressure, has been chosen to compare the performan- in hydrogen liquefaction, which requires cooling it down
ces of different thermodynamic models in predicting it to around 20 K.
with the aim of providing some guidelines for selecting The ZJ EoS is a modification of the original Redlich-Kwong
the most suitable model. This comparative analysis has (RK) EoS and it was developed to calculate phase equili-
23
been also extended to mixtures of hydrogen and na- bria in pure and multicomponent systems. It is particularly
20
tural gas based on vapor-liquid equilibrium (VLE) data accurate near and above the critical point of components
available in the literature. The most suitable EoS has, like light hydrocarbons, hydrogen, carbon dioxide and
then, been used to investigate the effect on some pro- hydrogen sulphide and it is recommended for systems
perties due to the addition of hydrogen to a natural gas containing high quantities of hydrogen to which special
mixture. treatment is given because of its quantum behavior.
80 80 Impiantistica Italiana - Gennaio-Febbraio 2022